For other uses, see Vaccine (disambiguation).
A vaccine is a biological preparation which is used to establish or improve immunity to a particular disease.
Vaccines can be prophylactic (e.g. to prevent or ameliorate the effects of a future infection by any natural or "wild" pathogen), or therapeutic (e.g. vaccines against cancer are also being investigated; see cancer vaccine).
Name and history
The term "vaccine" derives from Edward Jenner's 1796 use of cowpox (Latin variolæ vaccinæ, adapted from the Latin vaccīn-us, from vacca cow), which, when administered to humans, provided them protection against smallpox. The earliest vaccines were based on the concept of variolation originating in China, in which a person is deliberately infected with a weak form of smallpox as a form of inoculation. Jenner realized that milkmaids who had contact with cowpox did not get smallpox. The process of distributing and administrating vaccines is thus referred to as "vaccination" .
Jenner's work was continued by Louis Pasteur and others in the 19th century. Since vaccination against smallpox was much safer than smallpox inoculation, the latter fell into disuse and was eventually banned in England in 1849.
The 19th and 20th centuries saw the introduction of several successful vaccines against a number of infectious diseases. These included bacterial and viral diseases, but not (to date) any parasitic diseases.
Types
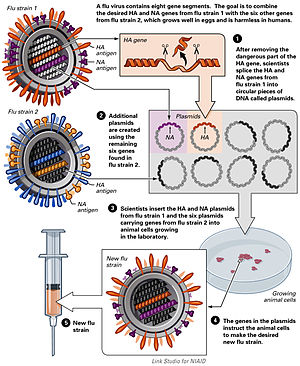
Avian Flu vaccine development by reverse genetics techniques.
Vaccines may be dead or inactivated organisms or purified products derived from them.
There are four types of traditional vaccines:[1]
Vaccines containing killed microorganisms - these are previously virulent micro-organisms which have been killed with chemicals or heat. Examples are vaccines against flu, cholera, bubonic plague, and hepatitis A.
Vaccines containing live, attenuated virus microorganisms - these are live micro-organisms that have been cultivated under conditions that disable their virulent properties or which use closely-related but less dangerous organisms to produce a broad immune response. They typically provoke more durable immunological responses and are the preferred type for healthy adults. Examples include yellow fever, measles, rubella, and mumps. The live tuberculosis vaccine is not the contagious strain, but a related strain called "BCG"; it is used in the United States very infrequently.
Toxoids - these are inactivated toxic compounds in cases where these (rather than the micro-organism itself) cause illness. Examples of toxoid-based vaccines include tetanus and diphtheria. Not all toxoids are for micro-organisms; for example, Crotalis atrox toxoid is used to vaccinate dogs against rattlesnake bites.
Subunit - rather than introducing an inactivated or attenuated micro-organism to an immune system (which would constitute a "whole-agent" vaccine), a fragment of it can create an immune response. Characteristic examples include the subunit vaccine against HBV that is composed of only the surface proteins of the virus (produced in yeast) and the virus-like particle (VLP) vaccine against human papillomavirus (HPV) that is composed of the viral major capsid protein.
A number of innovative vaccines are also in development and in use:
Conjugate - certain bacteria have polysaccharide outer coats that are poorly immunogenic. By linking these outer coats to proteins (e.g. toxins), the immune system can be led to recognize the polysaccharide as if it were a protein antigen. This approach is used in the Haemophilus influenzae type B vaccine.
Recombinant Vector - by combining the physiology of one micro-organism and the DNA of the other, immunity can be created against diseases that have complex infection processes
DNA vaccination - in recent years a new type of vaccine, created from an infectious agent's DNA called DNA vaccination, has been developed. It works by insertion (and expression, triggering immune system recognition) into human or animal cells, of viral or bacterial DNA. Some cells of the immune system that recognize the proteins expressed will mount an attack against these proteins and cells expressing them. Because these cells live for a very long time, if the pathogen that normally expresses these proteins is encountered at a later time, they will be attacked instantly by the immune system. One advantage of DNA vaccines is that they are very easy to produce and store. As of 2006, DNA vaccination is still experimental.
While most vaccines are created using inactivated or attenuated compounds from micro-organisms, synthetic vaccines are composed mainly or wholly of synthetic peptides, carbohydrates or antigens.
Vaccines may be monovalent (also called univalent) or multivalent (also called polyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism.[2] A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms.[3]
[edit] Developing immunity
The immune system recognizes vaccine agents as foreign, destroys them, and 'remembers' them. When the virulent version of an agent comes along the body recognises the protein coat on the virus, and thus is prepared to respond, by (1) neutralizing the target agent before it can enter cells, and (2) by recognizing and destroying infected cells before that agent can multiply to vast numbers.
Vaccines have contributed to the eradication of smallpox, one of the most contagious and deadly diseases known to man. Other diseases such as rubella, polio, measles, mumps, chickenpox, and typhoid are nowhere near as common as they were just a hundred years ago. As long as the vast majority of people are vaccinated, it is much more difficult for an outbreak of disease to occur, let alone spread. This effect is called herd immunity. Polio, which is transmitted only between humans, is targeted by an extensive eradication campaign that has seen endemic polio restricted to only parts of four countries.[2] The difficulty of reaching all children as well as cultural misunderstandings, however, have caused the eradication date to be missed several times.
[edit] Schedule
Main article: Vaccination schedule
See also: Vaccination policy
In order to provide best protection, children are recommended to receive vaccinations as soon as their immune systems are sufficiently developed to respond to particular vaccines, with additional 'booster' shots often required to achieve 'full immunity'. This has led to the development of complex vaccination schedules. In the United States, the Advisory Committee on Immunization Practices, which recommends schedule additions for the Center for Disease Control, recommends routine vaccination of children against: hepatitis A, hepatitis B, polio, mumps, measles, rubella, diphtheria, pertussis, tetanus, HiB, chicken pox, rotavirus, influenza, meningococcal disease and pneumonia. The large number of vaccines and boosters recommended (up to 24 injections by age two) has led to problems with achieving full compliance. In order to combat declining compliance rates, various notification systems have been instituted and a number of combination injections are now marketed (e.g., Prevnar and ProQuad vaccines), which provide protection against multiple diseases.
Besides recommendations for infant vaccinations and boosters, many specific vaccines are recommended at other ages or for repeated injections throughout life -- most commonly for measles, tetanus, influenza, and pneumonia. Pregnant women are often screened for continued resistance to rubella. The human papillomavirus vaccine is currently recommended in the U.S. and UK for ages 11–25. Vaccine recommendations for the elderly concentrate on pneumonia and influenza, which are more deadly to that group. In 2006, a vaccine was introduced against shingles, a disease caused by the chicken pox virus, which usually affects the elderly.
In Australia, a massive increase in vaccination rates was observed when the federal government made certain benefits (such as the universal 'Family Allowance' welfare payments for parents of children) dependent on vaccination. As well, children were not allowed into school unless they were either vaccinated or their parents completed a statutory declaration refusing to immunize them, after discussion with a doctor, and other bureaucracy. (Similar school-entry vaccination regulations have been in place in some parts of Canada for several years.) It became easier and cheaper to vaccinate one's children than not to. When faced with the annoyance, many more casual objectors simply gave in.[citation needed]
[edit] Efficacy
Vaccines do not guarantee complete protection from a disease. Sometimes this is because the host's immune system simply doesn't respond adequately or at all. This may be due to a lowered immunity in general (diabetes, steroid use, HIV infection) or because the host's immune system does not have a B-cell capable of generating antibodies to that antigen.
Even if the host develops antibodies, the human immune system is not perfect and in any case the immune system might still not be able to defeat the infection.
Adjuvants are typically used to boost immune response. Adjuvants are sometimes called the dirty little secret of vaccines [3] in the scientific community, as not much is known about how adjuvants work. Most often aluminium adjuvants are used, but adjuvants like squalene are also used in some vaccines and more vaccines with squalene and phosphate adjuvants are being tested. The efficacy or performance of the vaccine is dependent on a number of factors:
the disease itself (for some diseases vaccination performs better than for other diseases)
the strain of vaccine (some vaccinations are for different strains of the disease) [4]
whether one kept to the timetable for the vaccinations (see Vaccination schedule)
some individuals are 'non-responders' to certain vaccines, meaning that they do not generate antibodies even after being vaccinated correctly
other factors such as ethnicity or genetic predisposition
When a vaccinated individual does develop the disease vaccinated against, the disease is likely to be milder than without vaccination.
The following are important considerations in the effectiveness of a vaccination program:[citation needed]
careful modelling to anticipate the impact that an immunisation campaign will have on the epidemiology of the disease in the medium to long term
ongoing surveillance for the relevant disease following introduction of a new vaccine and
maintaining high immunisation rates, even when a disease has become rare.
In 1958 there were 763,094 cases of measles and 552 deaths in the United States.[4][5] With the help of new vaccines, the number of cases dropped to fewer than 150 per year (median of 56).[5] In early 2008, there were 64 suspected cases of measles. 54 out of 64 infections were acquired outside of the United States, and 63 of 64 either had never been vaccinated against measles, or were uncertain whether they had been vaccinated.[5]